The intricate interplay between tumors and the immune system has not only deepened our understanding of cancer biology but has also driven a paradigm shift from fundamental research to clinical immunotherapy, marking a milestone in modern oncology. As research continues to advance, accumulating evidence highlights the critical role of neural signaling in tumor initiation, progression, and invasion. Tumor neurobiology has thus emerged as a frontier field with significant implications for both basic research and clinical translation.
Perineural invasion (PNI), a pathological hallmark of aberrant tumor–nerve interactions, represents a key indicator of aggressive behavior in multiple solid tumors, including cholangiocarcinoma and pancreatic cancer. Clinically, PNI is closely associated with local recurrence, distant metastasis, and poor prognosis, making it one of the major pathological factors limiting long-term survival. Despite its clinical importance, the spatial organization and molecular regulatory mechanisms underlying PNI have remained poorly understood.
Recently, a research team led by Academician Dong Jiahong at the Hepatopancreatobiliary Center, Beijing Tsinghua Changgung Hospital, in collaboration with the Department of Pathology headed by Dr. Hongfang Yin, achieved a major breakthrough in the study of neural–tumor interactions in distal cholangiocarcinoma (dCCA). For the first time, the team applied Xenium subcellular-resolution spatial transcriptomics to systematically dissect the cellular and molecular architecture of the PNI microenvironment in dCCA, generating the first high-resolution spatial cellular atlas of perineural invasion in dCCA.
By integrating spatial transcriptomics with high-resolution pathological morphology, the study constructed a comprehensive PNI spatial atlas encompassing over 350,000 spatially resolved single cells across 20 major cell types. Within a unified tissue framework, the researchers achieved precise spatial registration of H&E staining, immunofluorescence imaging, and spatial transcriptomic signals, enabling an accurate reconstruction of key cellular interactions and molecular events within the perineural invasion niche.
Building on this atlas, the team systematically annotated all PNI-positive regions across pathological sections for each patient and introduced a patient-level Perineural Invasion Density Index (PNI density index) normalized to tumor size. This quantitative metric enabled refined stratification of patients based on PNI burden. Comparative analyses revealed that tumors with high PNI density exhibited significant upregulation of pathways related to neurogenesis, axon regeneration, extracellular matrix remodeling, and Schwann cell differentiation, compared with tumors showing low PNI involvement.
These findings suggest that tumor cells do not merely spread passively along nerve fibers but actively acquire neurotropic invasive capabilities through a process of “neural education,” providing new mechanistic insights into the aggressive behavior of distal cholangiocarcinoma and opening avenues for targeted therapeutic intervention.
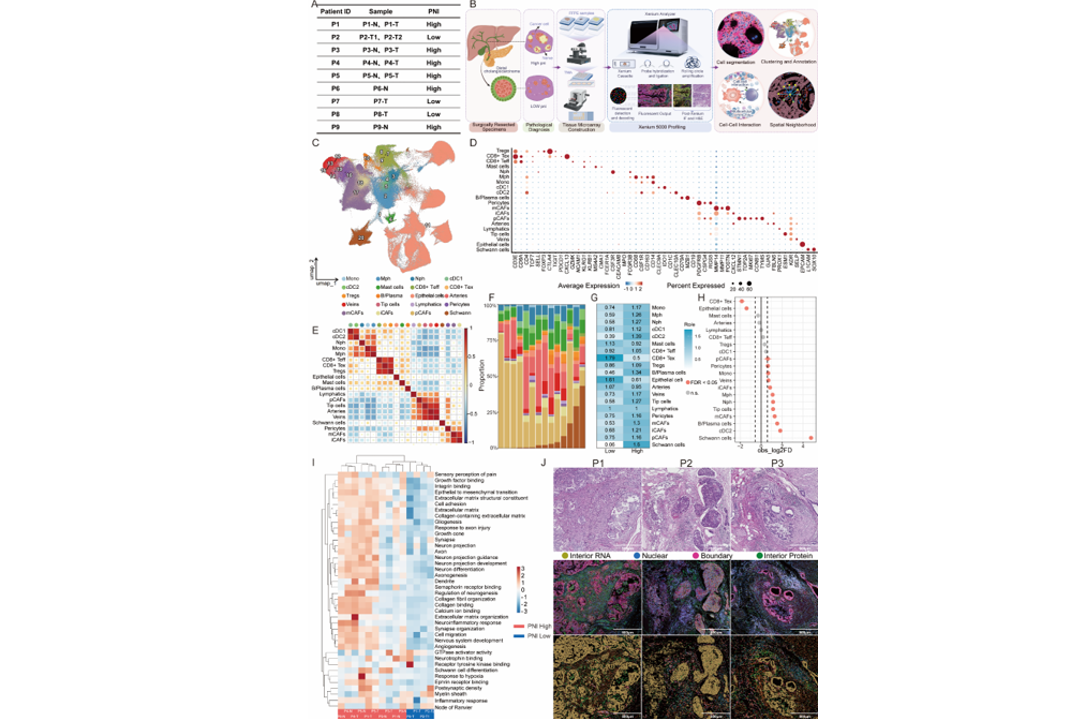
Meanwhile, the tumor microenvironment of patients with a high degree of perineural invasion exhibited significant enrichment of cDC2s, M2-polarized macrophages, myofibroblastic CAFs (mCAFs), and exhausted CD8⁺ T cells, indicating that perineural invasion is not an isolated pathological event but is accompanied by pronounced fibrotic remodeling and immunosuppressive states. At both spatial and cellular levels, these findings reveal the fundamental biological nature of perineural invasion as a driver of tumor immune evasion and stromal reprogramming.
Beyond advancing the mechanistic understanding of perineural invasion in dCCA from a spatial multi-omics perspective, this study also identifies potential molecular markers with clinical relevance for patient stratification, surgical navigation, and margin assessment. Several molecules, including CLDN18, ANXA5, MUC1, and CD59, were found to be significantly upregulated within perineural invasion lesions, highlighting their strong translational potential and providing a molecular basis for the visualization and targeted management of perineural invasion in biliary tract malignancies.
From study design to clinical translation, the project embodies the concept of “Precision Surgery,” originally proposed by Academician Dong Jiahong, representing another important example of the precision surgery paradigm applied to basic and translational research in hepatobiliary and pancreatic tumors.
The study, entitled “A Subcellular Spatial Atlas Illuminates the Microenvironmental Remodeling of Perineural Invasion in Distal Cholangiocarcinoma,” was published in the internationally renowned oncology journal Journal of Hematology & Oncology. Fansen Ji, a research-oriented postdoctoral fellow at the Hepatopancreatobiliary Center, Beijing Tsinghua Changgung Hospital; Hao Chen, a PhD candidate at the same center; and Huan Li, a pathologist, are co–first authors of the paper. Jiahong Dong, Xuedong Wang, Hongfang Yin, and Bingjun Tang serve as co-corresponding authors. The entire technical team of the Department of Pathology also made important contributions to this work.
This research was supported by the National Natural Science Foundation of China (NSFC) Integrated Project entitled “Visualization-Guided Precision Surgery Based on Key Molecular Functions of Perineural Invasion in Biliary Tract Malignancies.”
Editor: Li Han

